Tracking the dissolution of calcite single crystals in acid waters: a simple method for measuring fast surface kinetics
Abstract
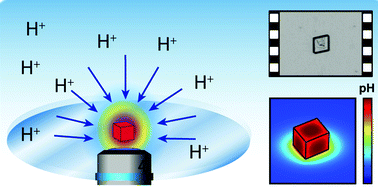
Although the dissolution kinetics of calcite in acid waters has been studied for more than a century, the process is not fully understood, and for particles and microcrystals the process is often assumed to be diffusion-controlled. Herein, the dissolution kinetics of calcite single microcrystals in aqueous solution (pH ca. 3) has been investigated for the first time by a combination of real-time optical microscopy coupled with numerical simulations. The small size and well-defined geometry of rhombohedral calcite single crystals enables the measurement of the dissolution rates of the individual crystal faces exposed to the solvent and an assessment of the relative importance of corners and edges compared to the {104} faces. Data are used to parameterise finite element method (FEM) models for the quantitative analysis of dissolution kinetics. The simulations provide an accurate determination of the near-interface concentration of solution species during dissolution, as well as concentration gradients. The intrinsic first-order dissolution rate constant for the attack of protons on the exposed {104} faces, ksurf = (6.4 ± 2.8) × 10−4 m s−1, is in good agreement with previous microscopic and macroscopic measurements, corroborating the method. This study is a further demonstration of the power of simple in situ optical microscopy for quantitative interfacial (dissolution/growth) kinetic measurements, using a configuration of practical relevance for processes as diverse as the remediation of acid water and scale removal.


 Please wait while we load your content...
Please wait while we load your content...