How silver segregation stabilizes 1D surface gold oxide: a cluster expansion study combined with ab initio MD simulations†
Abstract
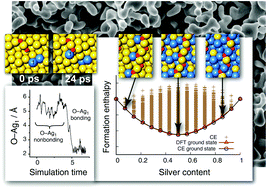
Gold has many unique properties, some of which continue to be uncovered, such as the rich chemistry of gold at the nanoscale. In this study, gold surprises us again by the unusual stability of one-dimensional gold oxide structures on the surface of gold, which enhances in the presence of silver impurities. We employ first-principles calculations to investigate the surface segregation of silver in the presence of atomic-oxygen adsorbates arranged in chains on the Au(321) surface. Such 1D oxide chains have previously been suggested as the most stable form of adsorbed oxygen on gold. Although Ag–O bonds are expected to be generally stronger than Au–O bonds, we show that this does not hold for 1D oxide chains, where Au–O bonds seem to be at least as strong as Ag–O bonds. Remarkably, we find that up to very high surface concentrations of silver, the Ag atoms do not occupy positions within the oxide chain, but prefer locations next to it. Ab initio molecular dynamics simulations support this picture and reveal how oxide chains and silver atoms rearrange on the surface toward a lower-energy configuration.


 Please wait while we load your content...
Please wait while we load your content...