Microelectrode voltammetry of multi-electron transfers complicated by coupled chemical equilibria: a general theory for the extended square scheme†
Abstract
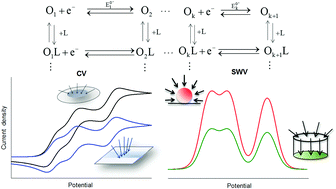
A very general and simple theoretical solution is presented for the current–potential–time response of reversible multi-electron transfer processes complicated by homogeneous chemical equilibria (the so-called extended square scheme). The expressions presented here are applicable regardless of the number of electrons transferred and coupled chemical processes, and they are particularized for a wide variety of microelectrode geometries. The voltammetric response of very different systems presenting multi-electron transfers is considered for the most widely-used techniques (namely, cyclic voltammetry, square wave voltammetry, differential pulse voltammetry and steady state voltammetry), studying the influence of the microelectrode geometry and the number and thermodynamics of the (electro)chemical steps. Most appropriate techniques and procedures for the determination of the ‘interaction’ between successive transfers are discussed. Special attention is paid to those situations where homogeneous chemical processes, such as protonation, complexation or ion association, affect the electrochemical behaviour of the system by different stabilization of the oxidation states.


 Please wait while we load your content...
Please wait while we load your content...