Vibrational Stark spectroscopy for assessing ligand-binding strengths in a protein
Abstract
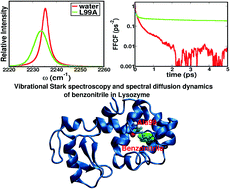
Nitrile groups are potentially useful spectroscopic probes in the infrared to characterize the binding and dynamics of ligands in proteins. This opens the possibility of locating and determining the binding mode of suitably labelled ligands in proteins based on optical spectroscopy, without the need for determining an X-ray structure. However, relating structure and spectroscopy requires means to accurately compute infrared spectra. This is investigated for benzonitrile (PhCN) in water, wild type (WT) and two lysozyme mutants in solution. The force field is validated by comparing with experimental data for benzonitrile in water which is the basis for computing the Stark shift and time scale for spectral diffusion of PhCN in WT and the L99A and L99G mutants of T4 lysozyme. The 1-d spectra for PhCN in WT and the two mutant proteins differ in their maximum absorption by up to 4 cm−1, which reflects the modified electrostatic environments in the three proteins. It is also tested whether extending from 1-d to 2-d infrared spectroscopy provides further discrimination in the ligand-binding modes. First, for PhCN in solution the frequency fluctuation correlation function (FFCF) decays to zero at short times whereas in the protein a pronounced static inhomogeneous component is found. Secondly, the decay time of the FFCF for the mutant to which PhCN binds most strongly has the longest decay time. It is demonstrated explicitly that the ligand-binding free energy with respect to the three protein variants correlates with the Stark shift. This makes 1-d infrared spectroscopy together with computations a valuable tool for characterizing binding modes and potentially binding locations in proteins.


 Please wait while we load your content...
Please wait while we load your content...