The dynamics of the Hg + Br2 reaction: elucidation of the reaction mechanism for the Br exchange reaction†
Abstract
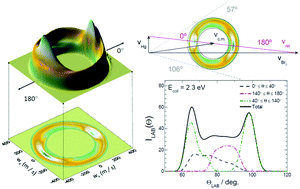
In spite of its importance in the Hg atmospheric chemistry, the dynamics of the Hg + Br2 → HgBr + Br reaction is poorly understood. In this article, we have carried out a comprehensive study of the reaction mechanism of this reaction by means of quasiclassical trajectories (QCTs) on an existing ab initio potential energy surface (PES). The reaction has a non trivial dynamics, as a consequence of its large endothermicity, the presence of a deep potential well, and the competition between the Br exchange and the collision induced dissociation processes. Our calculations demonstrate that insertion is only relevant at energies just above the reaction threshold and that, at energies above 2.3 eV, HgBr formation typically takes place via a sort of frustrated dissociation. In order to compare directly with the results obtained in extensive cross molecular beam experiments for the homologous reaction with I2, angular distributions in the laboratory frame for Hg + Br2 have been simulated under similar experimental conditions. The lack of agreement at the highest energies considered suggests that either the two reactions have substantially different mechanisms or that calculations on a single PES cannot account for the dynamics at those energies.


 Please wait while we load your content...
Please wait while we load your content...