Untangling surface oxygen exchange effects in YBa2Cu3O6+x thin films by electrical conductivity relaxation†
Abstract
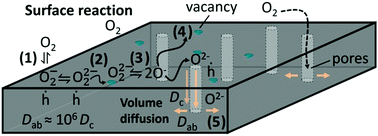
The kinetics of oxygen incorporation (in-diffusion process) and excorporation (out-diffusion process), in YBa2Cu3O6+x (YBCO) epitaxial thin films prepared using the chemical solution deposition (CSD) methodology by the trifluoroacetate route, was investigated by electrical conductivity relaxation measurements. We show that the oxygenation kinetics of YBCO films is limited by the surface exchange process of oxygen molecules prior to bulk diffusion into the films. The analysis of the temperature and oxygen partial pressure influence on the oxygenation kinetics has drawn a consistent picture of the oxygen surface exchange process enabling us to define the most likely rate determining step. We have also established a strategy to accelerate the oxygenation kinetics at low temperatures based on the catalytic influence of Ag coatings thus allowing us to decrease the oxygenation temperature in the YBCO thin films.


 Please wait while we load your content...
Please wait while we load your content...