Affinity regulation of the NH3 + H2O system by ionic liquids with molecular interaction analysis
Abstract
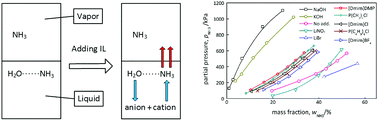
This work proposed using an adequate ionic liquid (IL) to weaken the affinity between NH3 and H2O as a potential solution to the issue of high-energy consumption involved in separating NH3 gas from liquid H2O. Two quaternary phosphonium-based ILs were selected according to an optimized regulation strategy. The regulation effects of the ILs were evaluated by the vapor–liquid equilibrium property of the NH3 + H2O + IL systems, and were compared with the regulation effects of traditional additives. The results showed that the expected effects were achieved by adding ILs. The regulation mechanisms of different strategies were discussed with respect to the molecular structure and chemical equilibrium for the first time limited to the authors' latest literature review. Finally, the IR spectra of the NH3 + H2O + IL systems were acquired and analyzed to verify the interactions of the ILs with NH3 and H2O.


 Please wait while we load your content...
Please wait while we load your content...