Supramolecular nanotubes based on halogen bonding interactions: cooperativity and interaction with small guests†
Abstract
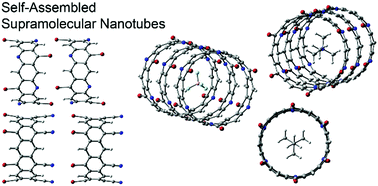
In this manuscript we study the formation of a series of self-assembled supramolecular nanotubes (SNTs) governed by noncovalent halogen bonding interactions. We have used eight different belts to construct the nanotubes, which are based on alternating benzene rings with 4-X-pyridine or p-X-benzonitrile rings (X = Br, I). The orientation of the halogen atoms can be parallel (denoted as “all up”) or antiparallel (denoted as “up/down”). In addition to the calculation of the formation energies of several binary and ternary assemblies, we have studied the ability of these non-covalently bonded self-organizing nanotubes to incorporate both anions and cations in their interior. We have used the BP86-D3/def2-TZVP level of theory to analyse the large assemblies and the RI-MP2/def2-TZVP level of theory for some model systems. This research emphasizes the role of σ-hole halogen bonds in the design and generation of assembled supramolecular structures and offers an approach for the creation of SNTs.


 Please wait while we load your content...
Please wait while we load your content...