Role of carboxylic acid groups in the reduction of nitric oxide by carbon at low temperature, as exemplified by graphene oxide†
Abstract
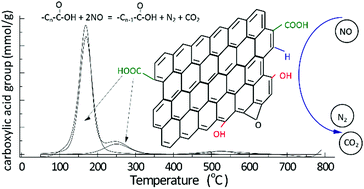
Graphene oxide (GO) was utilized to investigate the role of carboxylic acid groups in the reduction of nitric oxide (NO) for the first time. As a result, GO with sufficient carboxylic acid groups reduced 45% of NO at 100 °C. However, GO without these oxygen-containing groups barely reduced NO (lower than 5%) under the same conditions. After reduction of NO, the carboxylic acid group content on GO decreased from 8.32 to 5.22 mmol g−1. Simultaneously, the anhydride group content increased from 0.14 to 0.28 mmol g−1. FTIR spectroscopy also indicated that the carboxylic acid groups transformed into anhydride and lactone groups. Moreover, both transient kinetics and TG-MS studies demonstrated that reactive intermediates formed during the reaction between NO and GO at 100 °C. Based on these results, it was proposed that the carboxylic acid groups participated in NO reduction by consumption and regeneration. This mechanism explains why carbon is usually an effective reductant and catalyst support for NO removal at low temperature.


 Please wait while we load your content...
Please wait while we load your content...