Threshold concentration in the nonlinear absorbance law†
Abstract
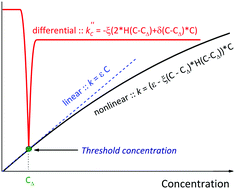
A new nonlinear relationship of the absorption coefficient with the concentration was proposed, allowing the calculation of the threshold concentration, which shows that there is a deviation from the Beer–Lambert law. The nonlinear model was successfully tested on a stable dimeric phthalocyanine ligand of J-type in solvents with different polarity. It was shown that deviation from the linearity is connected with a specific association of the macrocyclic molecules, which, in the case of non-polar solvents, leads to the formation of H-aggregates composed of J-type dimeric molecules. The aggregation number was estimated to be less than 1.5, which has allowed us to conduct a series of analytical experiments in a wide range of concentrations (1 × 10−6–5 × 10−4 mol L−1).


 Please wait while we load your content...
Please wait while we load your content...