Regular and red-shifted fluorescence of the donor–acceptor compound 5-(1H-pyrrole-1-yl)thiophenecarbonitrile (TCN) is efficiently quenched by internal modes of thiophene†
Abstract
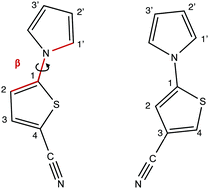
The photochemical properties of thiophene analogs of N-pyrrolobenzonitrile (PBN), notably the two isomers 5-(1H-pyrrole-1-yl)thiophene-2-carbonitrile (2-TCN) and 5-(1H-pyrrole-1-yl)thiophene-3-carbonitrile (3-TCN) have been investigated. The aim of this study is to reveal whether the donor–acceptor compound TCN shows a fluorescence behavior similar to other benzonitrile derivatives like PBN and N,N-dimethylaminobenzonitrile (DMABN). For this purpose high-level ab initio methods have been employed comprising approximate coupled cluster (CC2) methods, the algebraic-diagramatic construction scheme for the polarization propagator (ADC(2) and ADC(3)) as well as time-dependent density functional theory (TDDFT). Solvent effects have been included using continuum solvation models. In the gas phase excited TCN molecules most likely deactivate in a radiationless fashion to the ground state via a low lying S1/S0 conical intersection. In polar solvents the dark S(CT) (S2) state is stabilized below S(ππ*) (S1), however, despite this stabilization radiationless decay to the ground state remains the most likely deactivation pathway. Nevertheless, population of a twisted minimum of the S(CT) state becomes energetically feasible. Hence it is predicted that TCN does not show any fluorescence in the gas phase, and if at all only weak red-shifted emission from a twisted intramolecular charge transfer (TICT) minimum may be observable in polar solvents.


 Please wait while we load your content...
Please wait while we load your content...