Enhanced steady-state dissolution flux in reactive convective dissolution
Abstract
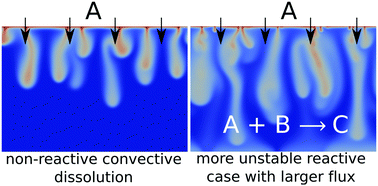
Chemical reactions can accelerate, slow down or even be at the very origin of the development of dissolution-driven convection in partially miscible stratifications when they impact the density profile in the host fluid phase. We numerically analyze the dynamics of this reactive convective dissolution in the fully developed non-linear regime for a phase A dissolving into a host layer containing a dissolved reactant B. We show for a general A + B → C reaction in solution, that the dynamics vary with the Rayleigh numbers of the chemical species, i.e. with the nature of the chemicals in the host phase. Depending on whether the reaction slows down, accelerates or is at the origin of the development of convection, the spatial distributions of species A, B or C, the dissolution flux and the reaction rate are different. We show that chemical reactions can enhance the steady-state flux as they consume A and can induce more intense convection than in the non-reactive case. This result is important in the context of CO2 geological sequestration where quantifying the storage rate of CO2 dissolving into the host oil or aqueous phase is crucial to assess the efficiency and the safety of the project.


 Please wait while we load your content...
Please wait while we load your content...