Unusual emission properties of the selected organosilicon compounds containing a styryl-carbazole chromophore: inversion of the singlet excited states†
Abstract
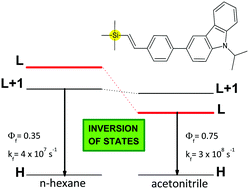
The spectroscopic and photophysical properties of silicon-containing styryl-carbazole were investigated in various solvents, and the results were analyzed with reference to its carbon derivatives. In n-hexane, both the silicon- and the carbon-containing compounds had very similar emission properties. In acetonitrile, the emission properties remained the same for the C-compound but changed significantly for the Si-compounds. In particular, the fluorescence spectra of the latter were red-shifted, and their radiative rate constants were even 7 times larger than in n-hexane, which suggested that the emissive states of the silicon-containing compounds were different in these two solvents. DFT calculations using the CAM-B3LYP functional showed that the emissive state of the C-compound involves the LUMO+1 orbital regardless of the medium. In contrast, for the Si-compound, changing the medium from n-hexane to acetonitrile resulted in the inversion of the emissive states from an excited state involving the LUMO+1 orbital (the dipole moment μ = 4.2 D) to an excited state involving the LUMO orbital (μ = 8.9 D).


 Please wait while we load your content...
Please wait while we load your content...