The charge transfer limit of a chemical adduct: the role of perturbation on external potential†
Abstract
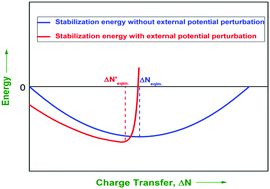
Full profiles of the components (positive and negative) of density functional reactivity theory (DFRT) based stabilization energy with respect to the amount of charge transfer (ΔN) are investigated on three different Diels–Alder pairs and twelve different charge transfer complexes formed by BH3–NH3 and their derivatives. One interesting observation is that the stabilization energy is zero when the charge transfer (ΔN) is either zero (lower limit, L.L.) or two times (higher limit, H.L.) the charge transfer at equilibrium (i.e., when chemical potentials are equalized). However, the existence of zero stabilization energy at the zero charge transfer limit is counter-argued after the inclusion of first and second order effects (due to a perturbing external potential of the partner of a given atom-in-a-molecule) in the individual energy components as well as the overall stabilization energy expressions. It has been shown that even when ΔN is zero (the lower limit), the net energy change is negative (i.e., the combined system is stabilized), highlighting the role of non-bonding interactions, rather than charge-transfer, in stabilizing the combined system at the initial stage of adduct formation. The higher limit (H.L.) of charge transfer is also shifted to a much lower value due to the inclusion of this external potential perturbation.

 Please wait while we load your content...
Please wait while we load your content...