Electrochemical reduction of CO2 on graphene supported transition metals – towards single atom catalysts†
Abstract
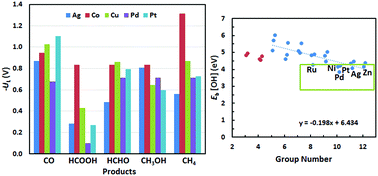
In this study, we have investigated the use of single metal atoms supported on defective graphene as catalysts for the electrochemical reduction of CO2 using the first-principles approach and the computational hydrogen electrode model. Reaction pathways to produce a variety of C1 products CO, HCOOH, HCHO, CH3OH and CH4 have been studied in detail for five representative transition metals Ag, Cu, Pd, Pt, and Co. Different pathways were revealed in contrast to those found for metallic crystalline surfaces and nanoparticles. These single atom catalysts have demonstrated a general improvement in rate limiting potentials to generate C1 hydrocarbons. They also show distinct differences in terms of their efficiency and selectivity in CO2 reduction, which can be correlated with their elemental properties as a function of their group number in the periodic table. Six best candidates for CH4 production are identified by conducting computational screening of 28 d-block transition metals. Ag has the lowest overpotential (0.73 V), and is followed by Zn, Ni, Pd, Pt and Ru with overpotentials all below 1 V. Cu in the supported single atom form shows a strong preference towards producing CH3OH with an overpotential of 0.68 V well below the value of 1.04 V for producing CH4.


 Please wait while we load your content...
Please wait while we load your content...