The effect of pressure on the crystallization of rapidly supercooled zirconium melts†
Abstract
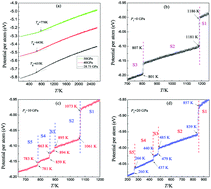
Molecular dynamics simulations have been performed to explore the effect of pressure (P) on the crystallization of zirconium (Zr) under rapid cooling. The structural evolutions have been analysed in terms of the system energy, the pair distribution function and the largest standard cluster analysis. It was found that at the cooling rate of 1.0 × 1011 K s−1, which can crystallize Zr melts into hcp crystals via the bcc intermediate state under zero pressure, the critical pressure (Pc) for vitrification is about 28.75 GPa, and the larger the pressure, the higher the glass transition temperature Tg. At P < Pc the Ostwald's step rule is applied to Zr melts. Crystallization of rapidly super-cooled Zr melts under pressure always begins with the bcc phase and ends in the hcp crystal; the higher the pressure, the lower the onset temperature (Tc) of crystallization. Unlike the single-intermediate-state crystallization (SisC) under zero pressure, multiple-intermediate-state crystallization (MisC) is usually observed under pressure. Structural analysis reveals that if nucleation is essentially completed at the end of the first crystalline (bcc-dominated) stage, MisC will occur; otherwise, SisC occurs. The origin of such an observation is also discussed from the effect of pressure upon the thermodynamics and kinetics factors. These findings are useful for comprehensively understanding the solidification of metals under pressure.


 Please wait while we load your content...
Please wait while we load your content...