Does water belong to the homologous series of hydroxyl compounds H(CH2)nOH?
Abstract
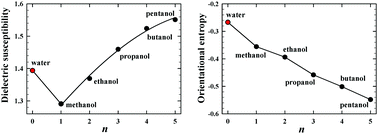
The main objective of this paper is to find a source of anomalously high value of the equilibrium permittivity of water. The source is identified to be the unusually high deformation polarizability. The conclusion follows from the analysis of the behavior of the orientational entropy increment induced by an external electric field applied to the liquids belonging to the homologous series of hydroxyl compounds H(CH2)nOH at the end of which water is located. The finding reflects the “indecision” of water about its dielectric relationship with the alcohol family: the value of the permittivity of water absolutely does not fit into alcohols (is too high), while the dipolar orientation effects (which normally determine the permittivity level) fit into alcohols quite well. It results from the presented experimental data that among all the diversity of intermolecular hydrogen-bonded structures existing in liquid water, predominant are the polar entities, i.e. the structures which more or less resemble the chains. Otherwise, the dipolar orientational effects would behave in a quite different way than what is observed in the experiment. The result is convergent with the conclusion of Wernet et al., based on the high-performance X-ray studies of water (Science, 2004).


 Please wait while we load your content...
Please wait while we load your content...