Nucleation-dependant chemical bonding paradigm: the effect of rare earth ions on the nucleation of urea in aqueous solution†
Abstract
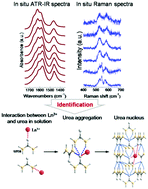
Rare earth ions can be used to construct a variety of novel structures and are favorable to chemical bonding regulation and design. In this study, the chemical bonding paradigm between rare earth ions (Ln3+) and urea molecules in an aqueous solution can be tracked by the evolution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH2, and CN vibration bands during the urea nucleation stage. Rare earth ions such as La3+, Gd3+, and Lu3+ can manipulate the nucleation time of urea via regulating the nucleation-dependant N–C
O, NH2, and CN vibration bands during the urea nucleation stage. Rare earth ions such as La3+, Gd3+, and Lu3+ can manipulate the nucleation time of urea via regulating the nucleation-dependant N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen-bonding between urea molecules. Two types of chemical bondings between Ln3+ and urea molecules have been confirmed, which are Ln3+⋯O
O⋯H–N hydrogen-bonding between urea molecules. Two types of chemical bondings between Ln3+ and urea molecules have been confirmed, which are Ln3+⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N and Ln3+⋯NH2−C. Compared with Ln3+⋯NH2−C, Ln3+ prefers to coordinate with the O
C–N and Ln3+⋯NH2−C. Compared with Ln3+⋯NH2−C, Ln3+ prefers to coordinate with the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in urea. With a higher concentration of rare earth ions in the solution, some N–C
C bond in urea. With a higher concentration of rare earth ions in the solution, some N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen bonds are broken as a consequence of the incorporation of Ln3+ into the lattice, resulting in the decreased symmetry of local urea molecules in the crystalline nuclei and the consequent Ln3+ concentration-dependent nucleation time of urea. Moreover, using the ionic electronegativity scale of Ln3+, the different effects of La3+, Gd3+, and Lu3+ on urea nucleation can be further distinguished. The present study provides basic data for unrevealing the chemical bonding regulation role of rare earth ions in the formation of hydrogen bonded materials, which may give insight into the design and fabrication of novel materials utilizing rare earth ions to adjust the chemical bonding process.
O⋯H–N hydrogen bonds are broken as a consequence of the incorporation of Ln3+ into the lattice, resulting in the decreased symmetry of local urea molecules in the crystalline nuclei and the consequent Ln3+ concentration-dependent nucleation time of urea. Moreover, using the ionic electronegativity scale of Ln3+, the different effects of La3+, Gd3+, and Lu3+ on urea nucleation can be further distinguished. The present study provides basic data for unrevealing the chemical bonding regulation role of rare earth ions in the formation of hydrogen bonded materials, which may give insight into the design and fabrication of novel materials utilizing rare earth ions to adjust the chemical bonding process.


 Please wait while we load your content...
Please wait while we load your content...