The mechanism of the Ser-(cis)Ser-Lys catalytic triad of peptide amidases
Abstract
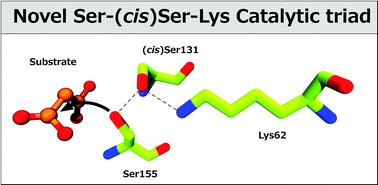
In this paper, we report a theoretical investigation of the catalytic mechanism of peptide amidases that involve a Ser-(cis)Ser-Lys catalytic triad. Previous suggestions propose that these enzymes should follow a distinct catalytic mechanism from the one that is present in the classic Ser-His-Asp catalytic triad. The theoretical and computational results obtained in this work indicate the opposite idea, showing that both mechanisms are very similar and only few differences are observed between both reactions. The results reveal that the different alignment of the Ser-(cis)Ser-Lys catalytic triad in relation to the classical Ser-His-Asp triad may provide a better stabilisation of the reaction intermediates, and therefore make these enzymes catalytically more efficient. The catalytic mechanism has been determined at the M06-2X/6-311++G**//B3LYP/6-31G* level of theory and requires five sequential steps instead of the two that are generally proposed: (i) nucleophilic attack of serine on the carbonyl group of the substrate, forming the first tetrahedral intermediate, (ii) formation of an acyl–enzyme complex, (ii) release of an ammonia product, (iv) nucleophilic attack of a water molecule forming the second tetrahedral intermediate, and (iv) the release of the product of the reaction, the carboxylic acid. The computational results suggest that the rate-limiting step is the first one that requires an activation free energy of 15.93 kcal mol−1. This result agrees very well with the available experimental data that predict a reaction rate of 2200 s−1, which corresponds to a free energy barrier of 14 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...