Unique agreement of experimental and computational infrared spectroscopy: a case study of lithium bromide solvation in an important electrochemical solvent
Abstract
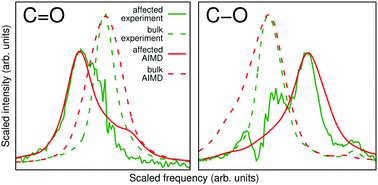
Infrared (IR) spectroscopy is a widely used and invaluable tool in the studies of solvation phenomena in electrolyte solutions. Using state-of-the-art chemometric analysis of a spectral series measured in a concentration-dependent manner, the spectrum of the solute-affected solvent can be extracted, providing a detailed view of the structural and energetic states of the solvent molecules influenced by the solute. Concurrently, ab initio molecular dynamics (AIMD) simulations provide the solvation shell picture at an atomistic detail level and allow for a consistent decomposition of the theoretical IR spectrum in terms of distance-dependent contributions of the solvent molecules. Here, we show for the first time how the chemometric techniques designed with the analysis of experimental data in mind can be harnessed to extract corresponding information from the computed IR spectra for mutual benefit, but without any mutual input. The wide applicability of this two-track approach is demonstrated using lithium bromide solvation in γ-butyrolactone (GBL) as a showcase. GBL is a cyclic ester with extensive applications as a solvent in electrochemistry and we are particularly motivated by its usefulness in the rechargeable cell industry which justifies further studies of lithium cation solvation in GBL. The combination of experiment and simulations firmly asserts the strong solvent structuring character of Li+ and a comparatively weak influence exerted on the solvent by Br−.


 Please wait while we load your content...
Please wait while we load your content...