Dangerous liaisons: anion-induced protonation in phosphate–polyamine interactions and their implications for the charge states of biologically relevant surfaces†
Abstract
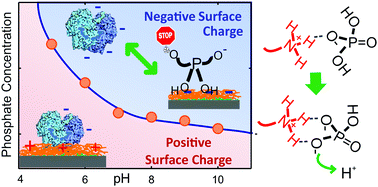
Although not always considered a preponderant interaction, amine–phosphate interactions are omnipresent in multiple chemical and biological systems. This study aims to answer questions that are still pending about their nature and consequences. We focus on the description of the charge state as surface charges constitute directing agents of the interaction of amine groups with either natural or synthetic counterparts. Our results allow us to quantitatively determine the relative affinities of HPO42− and H2PO4− from the analysis of the influence of phosphates on the zeta-potential of amino-functionalized surfaces in a broad pH range. We show that phosphate anions enhance the protonation of amino groups and, conversely, charged amines induce further proton dissociation of phosphates, yielding a complex dependence of the surface effective charge on the pH and phosphate concentration. We also demonstrate that phosphate–amine interaction is specific and the modulation of surface charge occurs in the physiological phosphate concentration range, emphasizing its biochemical and biotechnological relevance and the importance of considering this veiled association in both in vivo and in vitro studies.


 Please wait while we load your content...
Please wait while we load your content...