Hydrolysis of cephalexin and meropenem by New Delhi metallo-β-lactamase: the substrate protonation mechanism is drug dependent†
Abstract
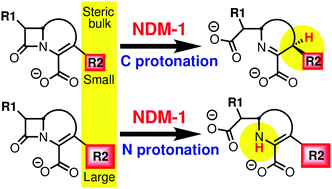
Emergence of antibiotic resistance due to New Delhi metallo-β-lactamase (NDM-1) bacterial enzymes is of great concern due to their ability to hydrolyze a wide range of antibiotics. There are ongoing efforts to obtain the atomistic details of the hydrolysis mechanism in order to develop inhibitors for NDM-1. In particular, it remains elusive how drug molecules of different families of antibiotics are hydrolyzed by NDM-1 in an efficient manner. Here we report the detailed molecular mechanism of NDM-1 catalyzed hydrolysis of cephalexin, a cephalosporin family drug, and meropenem, a carbapenem family drug. This study employs molecular dynamics (MD) simulations using hybrid quantum mechanical/molecular mechanical (QM/MM) methods at the density functional theory (DFT) level, based on which reaction pathways and the associated free energies are obtained. We find that the mechanism and the free energy barrier for the ring-opening step are the same for both the drug molecules, while the subsequent protonation step differs. In particular, we observe that the mechanism of the protonation step depends on the R2 group of the drug molecule. Our simulations show that allylic carbon protonation occurs in the case of the cephalexin drug molecule where Lys211 is the proton donor, and the proton transfer occurs via a water chain formed (only) at the ring-opened intermediate structure. Based on the free energy profiles, the overall kinetics of drug hydrolysis is discussed. Finally, we show that the proposed mechanisms and free energy profiles could explain various experimental observations.


 Please wait while we load your content...
Please wait while we load your content...