Kinetics, mechanisms and ionic liquids in the uptake of n-butylamine onto low molecular weight dicarboxylic acids
Abstract
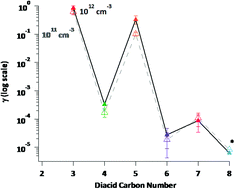
Atmospheric particles adversely affect visibility, health, and climate, yet the kinetics and mechanisms of particle formation and growth are poorly understood. Multiphase reactions between amines and dicarboxylic acids (diacids) have been suggested to contribute. In this study, the reactions of n-butylamine (BA) with solid C3–C8 diacids were studied at 296 ± 1 K using a Knudsen cell interfaced to a quadrupole mass spectrometer. Uptake coefficients for amines on the diacids with known geometric surface areas were measured at initial amine concentrations from (3–50) × 1011 cm−3. Uptake coefficients ranged from 0.7 ± 0.1 (2σ) for malonic acid (C3) to <10−6 for suberic acid (C8), show an odd–even carbon number effect, and decrease with increasing chain length within each series. Butylaminium salts formed from evaporation of aqueous solutions of BA with C3, C5 and C7 diacids (as well as C8) were viscous liquids, suggesting that ionic liquids (ILs) form on the surface during the reactions of gas phase amine with the odd carbon diacids. Predictions from the kinetic multi-layer model of aerosol surface and bulk chemistry (KM-SUB) were quantitatively consistent with uptake occurring via dissolution of the underlying diacid into the IL layer and reaction with amine taken up from the gas phase. The butylaminium salts formed from the C4 and C6 diacids were solids, and their uptake coefficients were smaller. These experiments and kinetic modeling demonstrate the unexpected formation of ILs in a gas–solid reaction, and suggest that ILs should be considered under some circumstances in atmospheric processes.


 Please wait while we load your content...
Please wait while we load your content...