Ag–Au bimetallic nanoclusters formed from a homogeneous gas phase: a new thermodynamic expression confirmed by molecular dynamics simulation†
Abstract
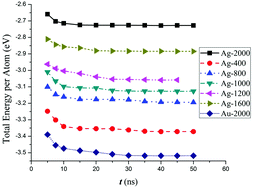
In this work, two probabilistic and thermodynamic limits for formation of a bimetallic nanocluster from a homogeneous gas phase were obtained in order to investigate the related phenomena using molecular dynamics simulation. Therefore, by application of some simple assumptions from thermodynamics and statistical mechanics, a new expression for composition of the nanocluster was derived which depends only on the initial conditions of the system and one adjustable parameter. This expression can be easily fitted to the results of molecular dynamics and can be used as a measure of the thermodynamic contribution in the cluster formation process. Then, molecular dynamics simulations were performed for several systems containing the same total number of metallic atoms and different concentrations of Ag and Au atoms. The results of this study exhibited that depending on different initial compositions of Ag and Au types, fcc and icosahedral structures are formed. Moreover, increase of the initial Ag concentration leads to products whose compositions are more controlled by probability limits. However, longer simulation times indicated that creation of more thermodynamically favoured nanoclusters depends on the formation of more probable ones in the early stages of the simulation.


 Please wait while we load your content...
Please wait while we load your content...