Dimer crystallization of chiral proteoids†
Abstract
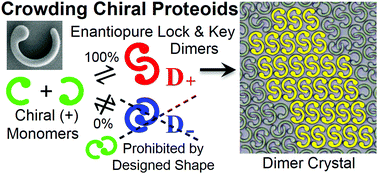
Proteins can self-assemble into a variety of exquisitely organized structures through hierarchical reaction pathways. To examine how different core shapes of proteins and entropy combine to influence self-assembly, we create systems of lithographically fabricated proteomimetic colloids, or ‘proteoids’, and explore how Brownian monolayers of mobile proteoids, which have hard interactions, self-assemble as they are slowly crowded. Remarkably, chiral C-shaped proteoids having circular heads on only one side form enantiopure lock-and-key chiral dimers; these dimers have corrugated, shape-complementary perimeters, so they, in turn, form lock-and-key arrangements into chiral dimer crystals. Time-lapse video microscopy reveals the expulsion of monomers from the growing dimer crystals through tautomerization translocation reactions which expedite the crystallization kinetics. By lithographically mutating proteoids, we also tune the types and structures of the resulting dimer crystals. Thus, rational design of sub-particle features in hard-core colloidal shapes can be used to sterically select desired self-assembly pathways without introducing any site-specific attractions, thereby generating a striking degree of hierarchical self-ordering, reminiscent of protein crystallization.


 Please wait while we load your content...
Please wait while we load your content...