Effects of the Hofmeister series of sodium salts on the solvent properties of water†
Abstract
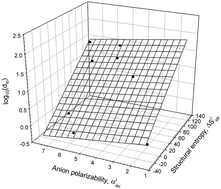
The solvent features of water (solvent dipolarity/polarizability, π*, hydrogen bond donor acidity, α, and hydrogen bond acceptor basicity, β) were examined in aqueous solutions of Na2SO4, NaF, CH3COONa, NaCl, NaBr, NaI, and NaClO4 at concentrations of each salt from 0 to 1.0 M (up to 2.0 M for NaClO4). The solvent features of water in solutions of different concentrations for each salt were found to be linearly related as π*ji = zjo + ajoαji + bjoβji. The coefficients of this relationship were suggested to represent the signature of the salt effect on the solvent features of water. The normalized distances for each salt were calculated using glucose as a reference compound. These distances may be used as the relative measures of the salt–water interactions. It is demonstrated that the distances for all salts examined are interrelated with structural water entropies and static polarizabilities of anions. It is shown that the distance may be used as a measure of the relative effects of salts on precipitation of ferric oxide, excessive chemical potential of propanol in salt solutions, surface tension, and viscosity. The distance represents the relative measure of the salt effect on the solvent features of water in a salt solution. The examples presented confirm that the approach used does enable us to characterize the differences between the effects of salts in the Hofmeister series on the properties of water.


 Please wait while we load your content...
Please wait while we load your content...