Alternative probe for the determination of the hydrogen-bond acidity of ionic liquids and their aqueous solutions†
Abstract
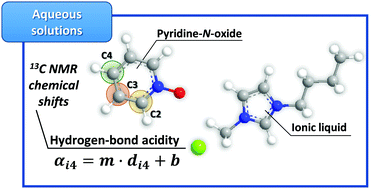
Although highly relevant to a priori select adequate solvents for a given application, the determination of the hydrogen-bond acidity or proton donor ability of aqueous solutions of ionic liquids is a difficult task due to the poor solubility of the commonly used probes in aqueous media. In this work, we demonstrate the applicability of the pyridine-N-oxide probe to determine the hydrogen-bond acidity of both neat ionic liquids and their aqueous solutions, based on 13C NMR chemical shifts, and the suitability of these values to appraise the ability of ionic liquids to form aqueous two-phase systems.


 Please wait while we load your content...
Please wait while we load your content...