Humidity-induced formation of water channels in supramolecular assemblies of wedge-shaped amphiphiles: the effect of the molecular architecture on the channel topology†
Abstract
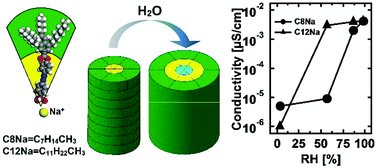
In supramolecular assemblies, absorption of water can assist the channel formation, similarly to biological systems and Nafion-like commercial ion-selective membranes. In this work, we investigate humidity-induced formation of water channels in wedge-shaped amphiphilic molecules, namely sodium 4′-[3′′,4′′,5′′-tris(alkyloxy)benzoyloxy]azobenzene-4-sulfonates. The studied molecules contain a polar sulfonate group at the tip and a hydrophobic periphery composed of alkyl chains of two different lengths. Upon increasing the relative humidity (RH) the amount of absorbed water significantly increases for the mesogen with dodecyl chains as compared to the one with octyl groups. In the former case, water sorption is accompanied by a considerable enhancement of ionic conductivity and a phase transition. In particular, an increase of RH induces a transition from a lamellar to a columnar phase resulting in the formation of 1D water channels running along the axis of the supramolecular columns. For the compound with shorter alkyl chains the lamellar phase exists in the entire RH-range exhibiting pronounced swelling at high RH-values and thereby forming a 2D water channel structure. NMR diffusometry was used to address the different molecular motions in the lyotropic mesophases of the studied amphiphiles.


 Please wait while we load your content...
Please wait while we load your content...