The phase and interfacial properties of azeotropic refrigerants: the prediction of aneotropes from molecular theory†
Abstract
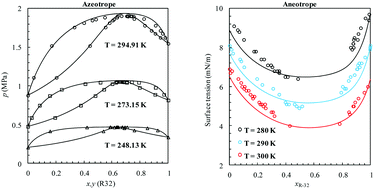
The use of hydrofluorocarbons (HFCs) as alternative non-ozone depleting refrigerants for chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) has grown during the last couple of decades. Owing to their considerable global warming potential, a global deal has been reached recently to limit the production and consumption of HFCs. For rational design of new refrigerants that are environmentally friendlier, the thermodynamics of current ones need to be well understood first. In this work, we examine the phase behavior of azeotropic refrigerants obtained by mixing HFCs with normal alkanes. The vapor–liquid equilibria (VLE) of these binary systems exhibit positive deviation from Raoult's law in the bulk, and a negative deviation from surface ideality (aneotrope) at the interface. The phase equilibria, second order thermodynamic derivative properties and interfacial properties of these complex systems were studied here using a modified version of the Statistical Associating Fluid Theory (SAFT) combined with Density Gradient Theory (DGT). The model was able to accurately capture the azeotropic nature of the phase equilibria and predict their composition and pressure at temperatures where experimental data are limited. In addition, accurate descriptions of the interfacial tensions were also obtained when compared with available experimental data, predicting the minimum found in surface tension as a function of composition. The molecular-based theory allowed the calculation of interfacial properties for which there is no experimental data available yet. Predictions show that the aneotrope occurs at a lower HFC composition for R-152a and R-134a systems in comparison to R-143a and R-125 systems. According to the calculated density profiles, HFC molecules appear to be preferentially adsorbed at the interface causing the surface tension of the n-alkane rich phase to decrease at low HFC concentrations. At high HFC concentrations, the phenomenon is inverted and n-alkane molecules are preferentially adsorbed causing the surface tension of the HFC rich phase to decrease. Consequently, the aneotrope point can be defined as the state at which the surface activity of both molecules is identical, or the relative adsorption of one component versus the other at the interface becomes zero.


 Please wait while we load your content...
Please wait while we load your content...