Adsorptive process design for the separation of hexane isomers using zeolites†
Abstract
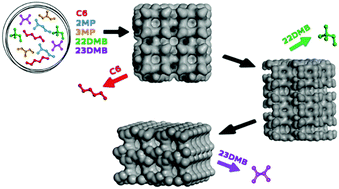
The product of catalytic isomerization is a mixture of linear and branched hydrocarbons that are in thermodynamic equilibrium, and their separation becomes necessary in the petrochemical industry. Zeolite 5A is usually industrially used to sieve alkane isomers, but its pore size allows only the separation of linear alkanes from the monobranched and dibranched alkanes by a kinetic mechanism. A more efficient approach to improve the average research octane number would be to adsorptively separate the di-methyl alkanes as products and recycle both the linear and mono-methyl alkanes to the isomerization reactor. Since the microscopic processes of adsorbates in zeolites are generally difficult or impossible to determine by experiments, especially in the case of mixtures, molecular simulation represents an attractive alternative. In this computational study, we propose a conceptual separation process for hexane isomers consisting of several adsorptive steps. Different zeolite topologies were examined for their ability to conduct this separation based on adsorption equilibrium and kinetics.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...