Anion-regulated electronic communication in a cyclometalated diruthenium complex with a urea bridge†
Abstract
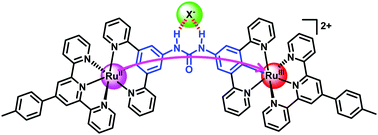
A combined study of electrochemical measurements, intervalence charge transfer analysis, and DFT calculations suggests that the degree of urea-mediated electronic coupling between two cyclometalated ruthenium sites is enhanced by the coordination of urea with Br− or Cl−via hydrogen bonding. In contrast, the redox waves of the diruthenium complex become highly irreversible in the presence of relatively strong basic anions such as H2PO4−, F−, or OAc−. This work demonstrates that the anion–urea interaction can be employed to regulate the electronic coupling and electron transfer between redox-active sites, suggesting the potential applications of the urea-functionalized diruthenium complex in anion sensing and stimuli-responsive molecular electronics.


 Please wait while we load your content...
Please wait while we load your content...