Determination of the equilibrium constant of C60 fullerene binding with drug molecules
Abstract
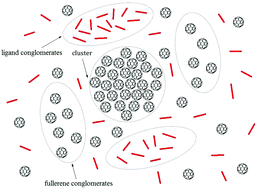
We report a new analytical method that allows the determination of the magnitude of the equilibrium constant of complexation, Kh, of small molecules to C60 fullerene in aqueous solution. The developed method is based on the up-scaled model of C60 fullerene–ligand complexation and contains the full set of equations needed to fit titration datasets arising from different experimental methods (UV-Vis spectroscopy, 1H NMR spectroscopy, diffusion ordered NMR spectroscopy, DLS). The up-scaled model takes into consideration the specificity of C60 fullerene aggregation in aqueous solution and allows the highly dispersed nature of C60 fullerene cluster distribution to be accounted for. It also takes into consideration the complexity of fullerene–ligand dynamic equilibrium in solution, formed by various types of self- and hetero-complexes. These features make the suggested method superior to standard Langmuir-type analysis, the approach used to date for obtaining quantitative information on ligand binding with different nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...