Trends in water-promoted oxygen dissociation on the transition metal surfaces from first principles†
Abstract
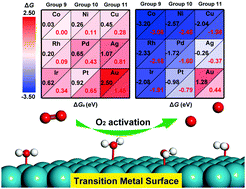
Dissociation of O2 into atomic oxygen is a significant route for O2 activation in metal-catalyzed oxidation reactions. In this study, we systematically investigated the mechanisms of O2 dissociation and the promoting role of water on nine transition metal (Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, and Au) surfaces. It was found that on clean metal surfaces, the dissociation of O2 was most favorable on Co(0001) and most difficult on Au(111), according to the free energy barriers of Co (0.03 eV) < Rh (0.20 eV) < Ni (0.26 eV) < Cu (0.45 eV) < Ir (0.62 eV) < Pd (0.65 eV) < Pt (0.92 eV) < Ag (1.07 eV) < Au (2.50 eV). With the involvement of water, O2 and H2O formed an O2⋯H2O complex via hydrogen bonding interactions, being accompanied by an increased co-adsorption free energy of 0.17–0.52 eV and a more activated O–O bond. More importantly, the introduction of water reduced the barriers of O2 dissociation on all the nine metal surfaces, with the reduction of the free energy barrier ranging from 0.03 eV on Co(0001) to 1.05 eV on Au(111). The intrinsic reasons for the promotional role of water are attributed to the hydrogen bonding effect between O2 and H2O and the electronic modification effect induced by the water–surface interaction. These results provide a fundamental understanding of the catalytic role of water in O2 dissociation on the transition metal surfaces and may be helpful in the rational design of new efficient catalysts for the oxidation reactions using molecular oxygen or air.


 Please wait while we load your content...
Please wait while we load your content...