Aggregation and insertion of melittin and its analogue MelP5 into lipid bilayers at different concentrations: effects on pore size, bilayer thickness and dynamics
Abstract
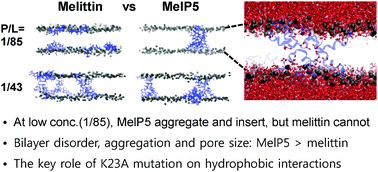
Melittin and its analogue MelP5 (five mutations T10A, R22A, K23A, R24Q, and Q26L of melittin) were simulated with lipid bilayers at different peptide/lipid molar ratios using all-atom and coarse-grained (CG) force fields. In CG simulations, both melittin and MelP5 insert into the bilayer at high concentration, while at low concentration only MelP5 can do so, showing the increased membrane permeability of MelP5 because five mutations weaken the electrostatic repulsion between peptides and strengthen the hydrophobic interactions between peptides and lipid tails, in quantitative agreement with experiments. In particular, aggregation of 6–8 MelP5 leads to pore formation, as also suggested by experiments. All-atom simulations, starting with atomic coordinates converted from the final configurations of CG simulations, show that MelP5 peptides bring more water molecules into the pores than do melittin peptides, indicating that MelP5 peptides form larger pores. Also, MelP5 peptides more effectively disorder lipids and thus increase the lateral mobility of lipids than do melittin peptides, leading to thinner bilayers. These findings indicate that differences of only five sequences can influence peptide aggregation and insertion, and bilayer thickness and dynamics, which helps explain experimental observations of the higher extent of antimicrobial activity and macromolecular leakage for MelP5 than for melittin, and also support experimental suggestions regarding the number of aggregated MelP5 and different effects of melittin and MelP5 on pore formation.


 Please wait while we load your content...
Please wait while we load your content...