Photo-catalyzed surface hydrolysis of iridium(iii) ions on semiconductors: a facile method for the preparation of semiconductor/IrOx composite photoanodes toward oxygen evolution reaction†
Abstract
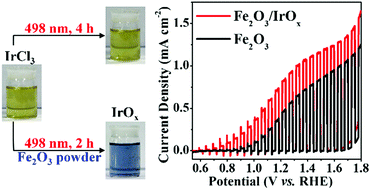
We previously reported that the hydrolysis of Ir3+ in homogeneous solution could be triggered by irradiation with light whose energy was larger than a threshold value. In this work, we demonstrated that, by introducing Fe2O3 particles into solution, the incident light energy-restriction for the photo-catalyzed hydrolysis could be broken and the hydrolysis occurred at the Fe2O3/solution interface. The photo-generated holes on the Fe2O3 surface played a key role in oxidizing Ir(III) to Ir(IV) species and triggered the deposition of IrOx. We showed that this photo-catalyzed surface hydrolysis is a universal phenomenon that takes place on the surface of many n-type semiconductors such as Fe2O3, TiO2, and Ag3PO4. As IrOx is an efficient catalyst for oxygen evolution reaction, surface hydrolysis is a general, facile and efficient strategy to prepare semiconductor/IrOx composites, which can be used as anodic materials for photoelectrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...