CO oxidation by the atomic oxygen on silver clusters: structurally dependent mechanisms generating free or chemically bonded CO2†
Abstract
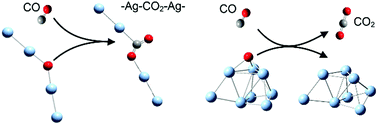
Atomic oxygen on silver is the crucial active species in many catalytic oxidation processes, while it is a big challenge to explore the relationship between its activity and molecular-level structures in condensed phases. We carried out kinetic measurements of the gas phase reactions between AgnO− (n = 1–8) and CO, in which the oxygen atoms were predicted to be terminal ones in AgO− and Ag2O−, in quasi-Ag–O–Ag chains for Ag3O− and Ag4O−, and on the two-fold or three-fold bridging positions in AgnO− (n = 5–8). All these oxygen species are highly reactive even at a low temperature of 150 K. AgnO− (n = 1, 2, 5–8) with terminal or bridging oxygen generate free CO2, while the quasi-chains of AgnO− (n = 3, 4) generate chemically bonded CO2 with a structural formula of Agn–CO2–Ag2− (n = 1, 2). Density functional theory calculations well interpreted all experimental observations, showing that no extra excitation energies are needed to initiate all these reactions. The structurally dependent mechanisms and the formation of chemically bonded CO2 revealed in this work help us to catch a glimpse of some important processes and intermediates on real silver catalysts.

 Please wait while we load your content...
Please wait while we load your content...