Benzimidazolylquinoxalines: novel fluorophores with tuneable sensitivity to solvent effects†
Abstract
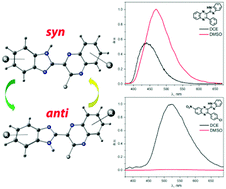
We report on the photophysical properties, conjugation, conformational behavior, intra- and intermolecular hydrogen bonds (HBs) of a series of novel fluorophores, consisting of 3-arylquinoxaline and benzimidazole moieties linked by a single CC bond. Computations employing density functional theory (DFT) reveal that conjugation between these moieties stabilizes syn-conformers with two HB centers located on the same side of the molecule. Anti-conformers form stronger intermolecular HBs with DMSO and DMF than syn-conformers, and this influences the energy gap between syn- and anti-forms, especially upon excitation of the molecules to the S1 state. Substituents introduced in various positions of the molecules modify their conformational behavior, and mutual disposition of excited singlet states relative to the ground states. Various substitution patterns produce very different effects on relative quantum yield of luminescence: from a moderate increase in polar DMSO and DMF relative to 1,2-dichloroethane solutions to complete quenching of emission which is observable in polar media. The observed behavior is understood with the aid of DFT and time-dependent DFT calculations. The tuneability of the spectroscopic range of the luminescence and especially of its sensitivity to environmental effects allows rational design of the novel fluorophores of this family for various applications.


 Please wait while we load your content...
Please wait while we load your content...