A first-principles study of the preventive effects of Al and Mg doping on the degradation in LiNi0.8Co0.1Mn0.1O2 cathode materials†
Abstract
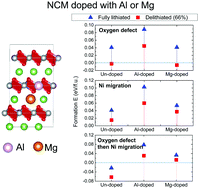
First-principles calculations have been used to investigate the effects of Al and Mg doping on the prevention of degradation phenomena in Li(Ni0.8Co0.1Mn0.1)O2 cathode materials. Specifically, we have examined the effects of dopants on the suppression of oxygen evolution and cation disordering, as well as their correlation. It is found that Al doping can suppress the formation of oxygen vacancies effectively, while Mg doping prevents the cation disordering behaviors, i.e., excess Ni and Li/Ni exchange, and Ni migration. This study also demonstrates that formation of oxygen vacancies can facilitate the construction of the cation disordering, and vice versa. Delithiation can increase the probabilities of formation of all defect types, especially oxygen vacancies. When oxygen vacancies are present, Ni can migrate to the Li site during delithiation. However, Al and Mg doping can inhibit Ni migration, even in structures with preformed oxygen defects. The analysis of atomic charge variations during delithiation demonstrates that the degree of oxidation behavior in oxygen atoms is alleviated in the case of Al doping, indicating the enhanced oxygen stability in this structure. In addition, changes in the lattice parameters during delithiation are suppressed in the Mg-doped structure, which suggests that Mg doping may improve the lattice stability.

 Please wait while we load your content...
Please wait while we load your content...