On the ionophoric selectivity of nonactin and related macrotetrolide derivatives†
Abstract
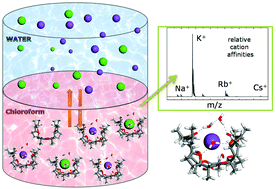
Nonactin and its analogs constitute a central class of macrocycles with an antibiotic activity closely related to their selective ionophoric behavior. In this study, we apply experimental and computational methods to revisit the specificity of cation binding and transport by three nactin variants differing in structural properties, such as the position of the ester linkages, the nature of the side groups, or the flexibility of the backbone. On the one hand, electrospray ionization mass spectrometry and infrared spectroscopy are employed to expose the selectivity of the liquid–liquid (water–chloroform) extraction of alkali cations by nonactin and to demonstrate that the cation complexes are partially hydrated in the organic phase. Furthermore, laser desorption mass spectrometry is employed to determine the intrinsic cation affinities of nonactin under solvent-free conditions. On the other hand, density functional theory calculations are performed to characterize the conformations of the alkali cation complexes of the three nactins, and to assess the role of intermolecular and solvent interactions in determining their relative stability. Depending on the structure of the macrocycle, the cation complexes adopt either a cage-like conformation or a tweezer-like conformation. The computations show that the partial hydration of those different conformations in the organic phase, determine the distinct cation extraction selectivities that are observed experimentally.

 Please wait while we load your content...
Please wait while we load your content...