Interfacial water on organic substrates at cryogenic temperatures: hydrogen bonding and quantification in the submonolayer regime
Abstract
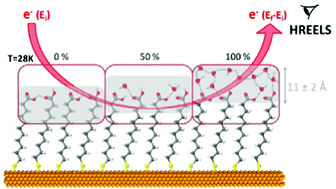
Water molecules were used to probe the physical and chemical properties of a model hydrophilic organic organized layer. To this end, H2O adsorption on mercaptoundecanoic acid self-assembled monolayers (SAMs) was investigated at the molecular level under ultra-high vacuum by high resolution electron energy loss spectroscopy (HREELS), through the sensitivity of the water OH stretching modes to the molecular environment. The water interfacial layer formation and structure were studied upon deposition at 28 K. A direct sensitive quantification in the submonolayer regime (10–80% of completion) was achieved by the sole measurement of the OH stretching mode frequencies, and the dominant basic (–COO−)/acidic (–COOH) forms of the terminal functions could be probed. The surface densities of the water interfacial layer and the SAM terminal functions were measured independently, and demonstrated to be comparable. This means that the SAM terminal functions provided anchors for water adsorption through two hydrogen bonds and that the SAM acted as a template even at 28 K. Upon annealing at 110 K, the water molecules were observed to form clusters of higher molecular density, dewetting the supporting substrate. Finally, the vanishing of the supporting substrate vibrational signature, due to the masking effect by the deposited water layer, was used to estimate the depth probed by HREELS through water layers to be 11 ± 2 Å.


 Please wait while we load your content...
Please wait while we load your content...