Molecular logic gates based on benzo-18-crown-6 ether of styrylquinoline: a theoretical study†
Abstract
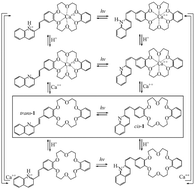
In the present work, we examine the possibility of a benzo-18-crown-6 ether of styrylquinoline molecule (1) in acetonitrile solvent to act as a sensor for the Ca++ cation and as a molecular logical gate. DFT and TDDFT calculations are carried out using the M06-2X and the PBE0 functionals. The quinoline moiety is an electron donor and an H+ receptor, while the crown ether is a Ca++ receptor forming host–guest complexes with Ca++. The calculations show that there are 8 thermally stable forms, i.e., trans and cis isomers of neutral (1), protonated (1H+), complexed with Ca++ (1Ca++), and both protonated and Ca++ complexed (1H+Ca++), with different absorption and emission spectra, and which can be interconverted from one form to another. The addition of H+ and/or Ca++ to 1 results in variation of the oscillator strength of the major absorption and emission peaks as well as in significant shifts of the major absorption and emission peaks including shifting from the vis spectral area to UV and vice versa. Consequently, 1 is a candidate for a sensor for the Ca++ cation. Furthermore it is shown that 1 can act as a molecular optical switch owing to its ability to be reversibly protonated and/or Ca++ complexed with substantial accompanying differences in the spectral properties. Similarly, 1 can be used as a sensor molecular logic gate, in which using H+ and Ca++ and irradiation as input, the emission output at 500, 470, 430, and 407 nm can be utilized as output to build AND, NOR, XOR, XNOR, INHIBIT, and IMPLICATION logic gates.

 Please wait while we load your content...
Please wait while we load your content...