DFT studies on the heterogeneous oxidation of SO2 by oxygen functional groups on graphene†
Abstract
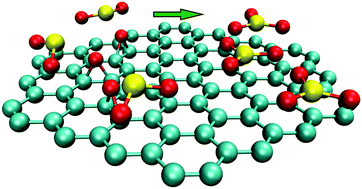
The heterogeneous oxidation of SO2 has been the subject of intense scrutiny in atmospheric chemistry because of the adverse effects of sulfate particles. Although it has been found that the soot particles with a graphene-like structure play an important role in the oxidation of SO2, little is known about the atomic-level mechanism involved. Here, we studied the oxidation of SO2 on oxygen-functionalized graphene using density functional theory (DFT) calculation. The results showed that SO2 is oxidized by the epoxide group via a two-step mechanism, where the C–O bond away from the SO2 is broken first, followed by the breaking of the other C–O bond and the synchronous formation of a new S–O bond. The energy barriers are significantly decreased when solvation free energies are involved, suggesting that humidity is favorable for promoting the oxidation by reducing the reaction barrier. The energy barriers for H2SO3 oxidation are much higher than that for SO2 oxidation, indicating that the direct conversion of SO2 to SO3 is the main pathway for the oxidation of SO2 by oxygen-functionalized graphene sheets in both the gas phase and solution. The reduced density gradient (RDG) analysis showed that the hydrogen bond formed between H2SO3 and epoxide groups enhances the stability of the reaction complex, and is responsible for the high energy barrier that has to be overcome for the reaction to proceed. These atomistic studies proposed a two-step mechanism for the oxidation of SO2 on the oxygen-functionalized graphene-like carbonaceous surfaces under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...