Reduced overpotentials for electrocatalytic water splitting over Fe- and Ni-modified BaTiO3
Abstract
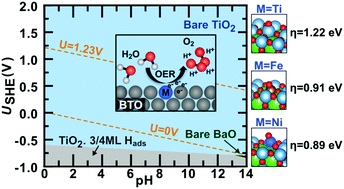
Water electrolysis is a key technology for the replacement of fossil fuels by environmentally friendly alternatives, but state-of-the-art water oxidation catalysts rely on rare elements such as Pt groups and other noble metals. In this article, we employ first-principles calculations to explore the potential of modified barium titanate (BaTiO3), an inexpensive perovskite oxide that can be synthesized from earth-abundant precursors, for the design of efficient water oxidation electrocatalysts. Our calculations identify Fe and Ni doping as a means to improve the electrical conductivity and to reduce the overpotential required for water oxidation over BaTiO3. Based on computed Pourbaix diagrams and pH/potential-dependent surface phase diagrams, we further show that BaTiO3 is stable under reaction conditions and is not sensitive with respect to poisoning by reaction intermediates and hydrogen adsorption. This proof of concept demonstrates that even minor compositional modifications of existing materials may greatly improve their catalytic activity, a fact that is often neglected when larger composition spaces are screened.


 Please wait while we load your content...
Please wait while we load your content...