A new sodiation–desodiation mechanism of the titania-based negative electrode for sodium-ion batteries†
Abstract
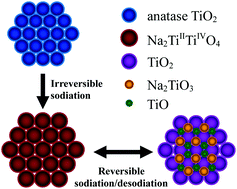
TiO2 is widely investigated as a negative electrode for lithium-ion batteries. In sodium-ion batteries, however, the sodiation–desodiation mechanism of TiO2 is still unclear. Here, we report a new sodiation–desodiation mechanism for an anatase TiO2/C electrode in an ionic liquid electrolyte at 90 °C, where it shows a high reversible capacity of 278 mA h g−1. During the first charge process, TiO2 reacts with Na ions to form a Na2TiIITiIVO4 solid solution. During the first discharge process, the solid solution converts into a mixture of TiO2, Na2TiO3, and TiO, with the former two being X-ray amorphous. In the subsequent cycle, the mixture acts as the active material, reversibly reacting with Na ions to re-form the Na2TiIITiIVO4 solid solution. This mechanism, which has not been reported for Na or Li ion insertion–extraction in anatase TiO2, can help understand this promising electrode material and develop safe sodium-ion batteries with high energy density.

 Please wait while we load your content...
Please wait while we load your content...