Characterization of N⋯O non-covalent interactions involving σ-holes: “electrostatics” or “dispersion”†
Abstract
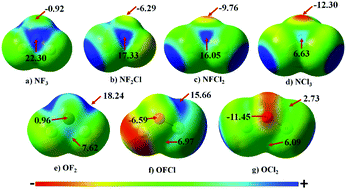
In this article, the existence of N⋯O noncovalent interactions was explored in per-halo substituted ammonia–water complexes. Optimized geometry at the MP2/aug-cc-pVTZ level shows that the N⋯O distance in all complexes is less than the sum of the vdW radii of N and O. The strength of these contacts was directly dependent on the extent of chlorine substitution on N or O atoms. Also, the level of theory and the basis set employed for the binding energy calculations have a direct effect on the strength of the N⋯O contacts. Energy decomposition analysis reveals that dispersion was the major contributor towards the stability of these contacts followed by electrostatic energy. The topological analysis further confirmed the existence of N⋯O contacts due to the presence of a bond critical point between the N and the O atom in all the complexes. These contacts have characteristics of a σ-hole interaction with the NBO analysis revealing that the primary charge transfer in all the complexes is occurring from O(lp) to σ*(N–X) orbitals, confirming these interactions to be predominantly in the category of pnicogen bonds.

 Please wait while we load your content...
Please wait while we load your content...