Nanosized Na-EMT and Li-EMT zeolites: selective sorption of water and methanol studied by a combined IR and TG approach†
Abstract
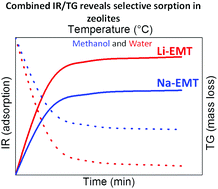
Nanosized EMT-type zeolite crystals in sodium (Na-EMT) and ion-exchanged lithium (Li-EMT) forms were prepared. The sorption behavior of Li(Na)-EMT samples towards water, methanol and a mixture of both (50 : 50) was studied by combined thermogravimetric and infrared spectroscopic methods. The stability of the samples prior to and after the sorption measurements in two subsequent cycles was confirmed by X-ray diffraction, N2 sorption and NMR spectroscopy. The high sorption capacity of the Li-EMT sample towards water was demonstrated. It was found that the methanol is replaced by water faster in the Li-EMT sample in comparison to the Na-EMT sample. At low temperature, the methanol shows weak adsorption on each cationic site and no side products during desorption for both samples were obtained.


 Please wait while we load your content...
Please wait while we load your content...