Atomic layer deposition of diisopropylaminosilane on WO3(001) and W(110): a density functional theory study†
Abstract
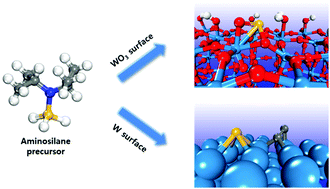
The decomposition reactions of the Si precursor, diisopropylaminosilane (DIPAS), on W(110) and hydroxylated WO3(001) surfaces are investigated to elucidate the initial reaction mechanism of the atomic layer deposition (ALD) process using density functional theory (DFT) calculations combined with ab initio molecular dynamics (AIMD) simulations. The decomposition reaction of DIPAS on WO3(001) consists of two steps: Si–N dissociative chemisorption and decomposition of SiH3*. It is found that the Si–N bond cleavage of DIPAS is facile on WO3(001) due to hydrogen bonding between the surface OH group and the N atom of DIPAS. The rate-determining step of DIPAS decomposition on WO3(001) is found to be the Si–H dissociation reaction of the SiH3* reaction intermediate which has an activation barrier of 1.19 eV. On the contrary, sequential Si–H dissociation reactions first occur on W(110) and then the Si–N dissociation reaction of the C5H7NSi* reaction intermediate is found to be the rate-determining step, which has an activation barrier of 1.06 eV. As a result, the final products in the DIPAS decomposition reaction on WO3(001) are Si* and SiH*, whereas Si* atoms remain with carbon impurities on W(110), which imply that the hydroxylated WO3 surface is more efficient for the ALD process.

 Please wait while we load your content...
Please wait while we load your content...