First-principles electronic structure calculations for the whole spinel oxide solid solution range MnxCo3−xO4 (0 ≤ x ≤ 3) and their comparison with experimental data†
Abstract
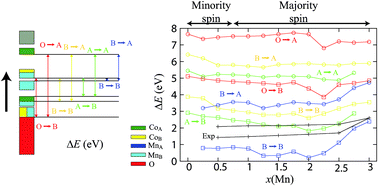
Transition metal spinel oxides have recently been suggested for the creation of efficient photovoltaic cells or photocatalysts. These compounds can be easily tuned by doping to adapt their electronic or magnetic properties. However, their cation distribution is very complex and band structures are still a subject of controversy. We propose a complete density functional theory investigation of MnxCo3−xO4 compounds, using different approximations in order to explain the variation of these properties as a function of composition (for 0 ≤ x ≤ 3) and determine the electronic structure over the whole solid solution range. A detailed study of their atomic structure, magnetic properties and electronic structure is given and compared with experimental data. The unit cell volume calculated for each composition is in agreement with the volume obtained experimentally in ceramics, while a cubic-to-tetragonal structural transition is predicted at x = 2.0. An antiferromagnetic to ferrimagnetic behavior is observed at the lowest ordering temperature depending on the composition. The band gap, deduced from our band structure calculations, strongly decreases upon doping of the end members Co3O4 and Mn3O4, but is partly restored by the tetragonal distortion. A direct band gap, close to 0.5–0.8 eV, is calculated for 0.25 ≤ x ≤ 2.25, justified by inter-metal transitions from Mn ions on octahedral sites.

 Please wait while we load your content...
Please wait while we load your content...