Gradient doping – a case study with Ti-Fe2O3 towards an improved photoelectrochemical response
Abstract
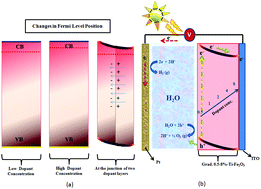
The present study investigates the effect of gradient doping on modifying the photoelectrochemical response of Ti-doped Fe2O3 photoanodes for their use in sunlight based water splitting for hydrogen evolution. The deposition of a thin film over the ITO (tin doped indium oxide) substrate was carried out using a spray pyrolysis method. The concentration of dopant was varied from 0.5–8.0 at% and two sets of samples were also prepared with low to high (0.5–8%) and high to low (8–0.5%) dopant concentrations in the direction towards the substrate. The prepared thin films were characterized using X-ray Diffractometry (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray (EDX) Spectroscopy, Secondary Ion Mass Spectroscopy (SIMS), X-ray Photoelectron Spectroscopy (XPS) and UV-visible Spectroscopy. The photoelectrochemical studies revealed that the deposition of dopant layers with a low to high concentration towards the substrate exhibited a highly improved photoresponse (200 times) in comparison to the pristine sample and a two fold enhancement in comparison to 2% Ti-doped Fe2O3. The improvement in the photoresponse has been attributed to the values of a high flat band potential, low resistance, high open circuit voltage, carrier separation efficiency, applied bias photon-to-current conversion efficiency (ABPE), and incident photon-to-current conversion efficiency (IPCE). A reduced charge transfer resistance has been demonstrated with Nyquist plots.


 Please wait while we load your content...
Please wait while we load your content...