Tunneling chemical exchange reaction D + HD → D2 + H in solid HD and D2 at temperatures below 1 K
Abstract
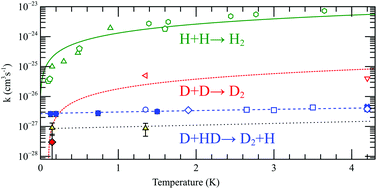
We report on a study of the exchange tunneling reaction D + HD → D2 + H in a pure solid HD matrix and in a D2 matrix with a 0.23% HD admixture at temperatures between 130 mK and 1.5 K. We found that the exchange reaction rates, kexHD ∼ 3 × 10−27 cm3 s−1 in the pure HD matrix, and kexD2 = 9(4) × 10−28 cm3 s−1 in the D2 matrix, are nearly independent of temperature within this range. This confirms the quantum tunnelling nature of these reactions, and their ability to proceed at temperatures down to absolute zero. Based on these observations we concluded that exchange tunneling reaction H + H2 → H2 + H should also proceed in a H2 matrix at the lowest temperatures. On the other hand, the recombination of H atoms in solid H2 and D atoms in solid D2 is substantially suppressed at the lowest temperatures as a result of a decreased probability of resonant tunneling of atoms when they approach each other.


 Please wait while we load your content...
Please wait while we load your content...